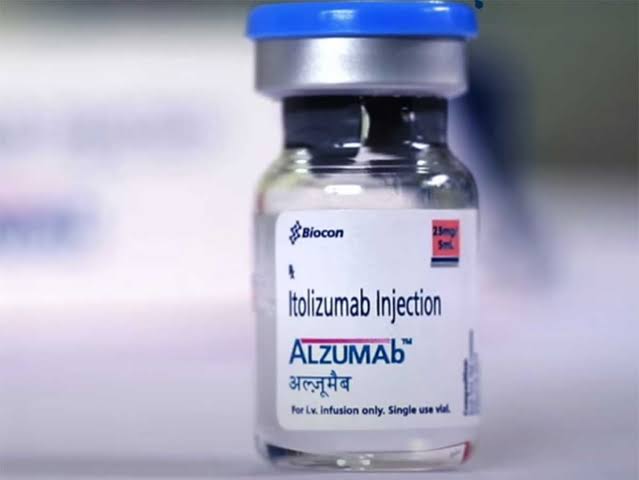
The biopharmaceutical company, Biocon, saw a huge sell-off on Monday following the announcement by the Union Ministry of Health to not include Itolizumab drug for Covid-19 treatment.
The Health Ministry said in a note that the National Task Force on Covid-19, constituted by the ministry, had said “there is little evidence in favour of this drug.” Biocon’s use of just 30 patients was also questioned by several independent experts. But Biocon noted that it is a drug that was previously approved for psoriasis, and was only a label extension. It also stated that emergency procedures to obtain approval for use on Covid-19 patients were following international norms.
Allegedly, Biocon also responded to the ministry’s announcement, stating that the National COVID task force needed to see more evidence and that the company will provide them large real-world data to enable the committee to reconsider its decision on the inclusion of Itolizumab in the protocol.
According to Biocon, approximately 1,000 patients have reportedly used the drug Itolizumab across India.
About two weeks ago, the Drug Controller General of India allowed the use of the drug for severe coronavirus patients, raising the company’s share price.