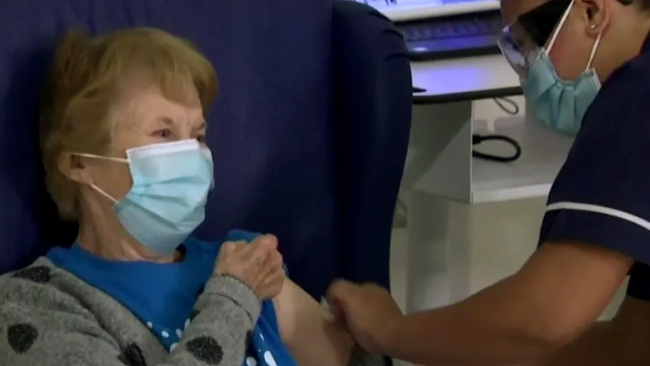
Margaret Keenan, a grandmother of four, made history on Tuesday after getting a potentially lifesaving birthday present. With one-shot or “jab” as Britons might say Keenan, who turns 91 next week, officially launched the United Kingdom’s nationwide coronavirus immunization campaign, the largest such effort in its history.
“I feel so privileged to be the first person vaccinated against COVID-19,” said Keenan, who received the shot at 6:30 a.m. U.K. time. “It is the best early birthday present I could wish for because it means I can finally look forward to spending time with my family and friends in the new year after being on my own for most of the year.”
The U.K.’s vaccination effort begins nearly a year after the virus first appeared in China and at the same time much of the world still grapples with the pandemic. British health officials said that Tuesday marked a turning point in the country’s battle with the virus, an illness that, as per John Hopkin’s University data has infected more than 67 million people around the world and has killed more than 1.5 million people. The U.K. alone has more than 1.7 million confirmed COVID-19 cases.
Health officials began the immunization campaign just six days after granting the vaccine’s makers, Pfizer and BioNTech, emergency approval. Britain’s government has secured 800,000 doses of the vaccine so far, enough to give 400,000 people two doses each, the government said. To immunize the rest of the country’s 68 million people, health officials must contend with enormous logistical challenges. Vials of the vaccine must be kept frozen at minus 70 degrees Celsius (minus 94 degrees Fahrenheit).
There are also concerns over how Britain’s impending split from the European Union could affect the vaccine’s transport from Belgium. The government said that it was prepared to ask the military to fly the vials over the border. The U.K. will give the vaccine first to front-line health workers, long-term care facility workers, and residents over 80 years old. Shots will be made available first at hospitals before being distributed to doctor’s offices.
National Health Service England’s chief executive Sir Simon Stevens said, “It will take some months to complete the work as more vaccine supplies become available and until then we must not drop our guard. But if we all stay vigilant in the weeks and months ahead, we will be able to look back at this as a decisive turning point in the battle against the virus.”
Meanwhile, the U.S. and other European countries are expected to approve the vaccine’s emergency use later this month. Pfizer’s coronavirus vaccine has proved to be nearly 95% effective in clinical trials. But the immunization campaign in the U.S. could be hurt by critical supply issues.
The New York Times reported that several months ago, the Trump administration passed on an option from Pfizer to purchase more vaccine doses beyond the initial 100 million agreed upon. Meanwhile, the Washington Post reported that the administration has been told by Pfizer that it cannot provide more vaccine until late June or July due to its commitments to other countries.
China and Russia have begun a mass rollout of their coronavirus vaccines before clinical tests are complete, in what is emerging as an unexpectedly complex geopolitical challenge for the United States. China’s Sinopharm announced this week that it would provide emergency doses of one of its two trial vaccines to the United Arab Emirates, prioritizing the U.S. ally over the vast majority of Chinese. China is now the sole supplier of Coronavirus vaccine to the Middle East.
Meanwhile, Russia’s sovereign wealth fund signed a deal to supply India with 100 million doses of the Sputnik V vaccine. These moves have thrown Western policymakers off balance. American health-care experts say that the United States should not rush out its own vaccine in response. But that leaves China and Russia as the only countries wielding this valuable diplomatic tool for potentially months to come.
The upshot is that by next year, China and Russia may have purchased significant geopolitical power by having bent the rules and rushed out their vaccines. It is also possible that their vaccines may fail, at an enormous human cost.
Hundreds of thousands of people in China, including diplomats, the military, front-line health workers , and employees of state-owned enterprises, have received Sinopharm’s vaccines under urgent-use stipulations, according to state media reports. But even as the rest of the country awaits access, Beijing has begun deploying vaccines abroad to regions where it is seeking to expand its influence.
Aside from the UAE collaboration, Sinopharm is also running Phase 3 trials in Jordan and Bahrain. On September 11, Egypt announced that it will also begin trials with Sinopharm, three days after the British-Swedish drugmaker AstraZeneca paused its clinical trial because of a “potentially unexplained illness.” The trial has since resumed, although not in the United States. Egypt signed a deal with AstraZeneca in July to purchase 30 million doses of its vaccine.
Officials in Moscow said that initial doses of the Sputnik V vaccine have been delivered to all regions of Russia, with health-care workers and teachers to be the first to receive access.